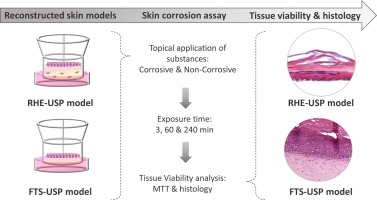
Study Design of In Vitro Eye Irritation EpiOcular (OECD 492) Study Design
Study Design of In Vitro Eye Irritation EpiOcular (OECD 492) Study Design The EpiOcular™ Eye Irritation Test (OECD 492) is an in vitro test method used to assess the acute eye irritation potential of chemicals and mixtures. It utilizes a reconstructed human corneal epithelium (RhCE) model to measure cytotoxicity, allowing for the identification of substances […]
Study Design of In Vitro Eye Irritation EpiOcular (OECD 492) Study Design Read More »


![In Vitro Skin Sensitization [Human Cell Line (U937-U-SENS) Activation Test (h-CLAT)] (OECD 442E) 4 In Vitro Skin Sensitization [Human Cell Line (U937-U-SENS) Activation Test (h-CLAT)] (OECD 442E)](https://www.scs-groups.com/wp-content/uploads/2024/12/In-Vitro-Skin-Sensitization-Human-Cell-Line-U937-U-SENS-Activation-Test-h-CLAT-OECD-442E-2048x1365-1-1024x683.jpg)
![Study Design of In Vitro Skin Sensitization - ARE-Nrf2 Luciferase Test Method [KeratinoSens] (OECD 442D) 7 Study Design of In Vitro Skin Sensitization - ARE-Nrf2 Luciferase Test Method [KeratinoSens] (OECD 442D)](https://www.scs-groups.com/wp-content/uploads/2024/12/In-Vitro-Skin-Sensitization-ARE-Nrf2-Luciferase-Test-Method-KeratinoSens.png)
![Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C) 10 Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C)](https://www.scs-groups.com/wp-content/uploads/2024/12/Study-Design-of-In-Vitro-Skin-Sensitization-Dermal-Peptide-Reactivity-Assay-DPRA-OECD-442C.png)

![Study Design of In Vitro Eye Irritation [Bovine Corneal Opacity and Permeability (BCOP) Test] (OECD 437) 16 Study Design of In Vitro Eye Irritation [Bovine Corneal Opacity and Permeability (BCOP) Test] (OECD 437)](https://www.scs-groups.com/wp-content/uploads/2024/12/In-Vitro-Eye-Irritation-Bovine-Corneal-Opacity-and-Permeability-BCOP-Test.jpeg)