Blog - In Vitro Studies
 In Vitro Studies
In Vitro Studies In Vitro Cytotoxicity in Balb/c 3T3 LD50 (OECD 129)
In Vitro Cytotoxicity in Balb/c 3T3 LD50 (OECD 129) The in vitro cytotoxicity assay in Balb/c 3T3 cells with an LD50 endpoint (OECD 129) is a test method used to determine the cytotoxic potential of chemicals towards mammalian cells. This information can be helpful
Read More In Vitro Studies
In Vitro Studies Study Design of In Vitro Skin Absorption: Franz Cell Diffusion Assay (OECD 428)
Study Design of In Vitro Skin Absorption: Franz Cell Diffusion Assay (OECD 428) The Franz Cell Diffusion Assay (OECD 428) is a well-established and standardized in vitro method for assessing the percutaneous absorption of chemicals through human skin. It mimics the passive diffusion of chemicals across
Read More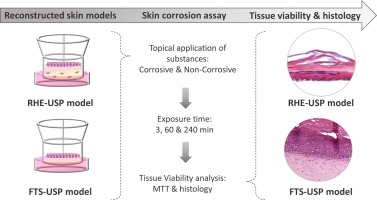 In Vitro Studies
In Vitro Studies Study Design of EpiSkin-In Vitro Skin Corrosion (OECD 431)
Study Design of EpiSkin-In Vitro Skin Corrosion (OECD 431) The EpiSkin-In Vitro Skin Corrosion Test, based on the OECD Guideline 431, is a validated, non-animal alternative for assessing the corrosive potential of chemicals and mixtures. It utilizes reconstructed human epidermis
Read More![Blog - In Vitro Studies 4 Study Design of In Vitro Eye Irritation [Bovine Corneal Opacity and Permeability (BCOP) Test] (OECD 437)](https://www.scs-groups.com/wp-content/uploads/2024/12/In-Vitro-Eye-Irritation-Bovine-Corneal-Opacity-and-Permeability-BCOP-Test.jpeg) In Vitro Studies
In Vitro Studies Study Design of In Vitro Eye Irritation [Bovine Corneal Opacity and Permeability (BCOP) Test] (OECD 437)
Study Design of In Vitro Eye Irritation [Bovine Corneal Opacity and Permeability (BCOP) Test] (OECD 437) The In Vitro Eye Irritation Test using the Bovine Corneal Opacity and Permeability (BCOP) method, outlined in OECD Guideline 437, is an alternative to
Read More In Vitro Studies
In Vitro Studies Study Design of EpiSkin-In Vitro Skin Irritation (OECD 439)
Study Design of EpiSkin-In Vitro Skin Irritation (OECD 439) The EpiSkin-In Vitro Skin Irritation Test, based on the OECD Guideline 439, is another validated alternative to animal testing for assessing the irritating potential of chemicals and mixtures. It utilizes reconstructed
Read More![Blog - In Vitro Studies 6 Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C)](https://www.scs-groups.com/wp-content/uploads/2024/12/Study-Design-of-In-Vitro-Skin-Sensitization-Dermal-Peptide-Reactivity-Assay-DPRA-OECD-442C.png) In Vitro Studies
In Vitro Studies Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C)
Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C) The Dermal Peptide Reactivity Assay (DPRA) is an in chemico assay used to assess the skin sensitization potential of chemicals. It is based on the principle that skin
Read MoreLinkedin Link
Medical Device Testing
Laboratory Testing

SCS Blog Category
Blog - ACUTE TOXICITY STUDIES
Blog - Biodegradability Testing – Chemical Impact
Blog - Environmental Fate & Toxicology Testing
Blog - In Vitro Studies
Blog - Laboratory Testing & Analysis
Blog - Medical Devices
Blog - OECD GLP
Blog - Scientific

JOIN WITH MANY WHO HAVE BENEFITED WITH US
SCS Consulting Group

