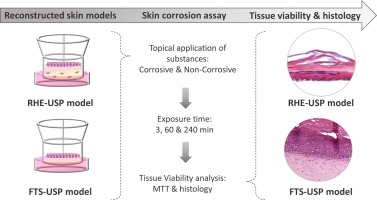
In Vitro Skin Sensitization [Human Cell Line (U937-U-SENS) Activation Test (h-CLAT)] (OECD 442E)
In Vitro Skin Sensitization [Human Cell Line (U937-U-SENS) Activation Test (h-CLAT)] (OECD 442E) The Human Cell Line Activation Test (h-CLAT) is another promising in vitro method for predicting skin sensitization potential, addressing the third key event in the Adverse Outcome Pathway (AOP): dendritic cell activation. As with the other assays you mentioned, it offers an alternative […]

![In Vitro Skin Sensitization [Human Cell Line (U937-U-SENS) Activation Test (h-CLAT)] (OECD 442E) 1 In Vitro Skin Sensitization [Human Cell Line (U937-U-SENS) Activation Test (h-CLAT)] (OECD 442E)](https://www.scs-groups.com/wp-content/uploads/2024/12/In-Vitro-Skin-Sensitization-Human-Cell-Line-U937-U-SENS-Activation-Test-h-CLAT-OECD-442E-2048x1365-1-1024x683.jpg)
![Study Design of In Vitro Skin Sensitization - ARE-Nrf2 Luciferase Test Method [KeratinoSens] (OECD 442D) 4 Study Design of In Vitro Skin Sensitization - ARE-Nrf2 Luciferase Test Method [KeratinoSens] (OECD 442D)](https://www.scs-groups.com/wp-content/uploads/2024/12/In-Vitro-Skin-Sensitization-ARE-Nrf2-Luciferase-Test-Method-KeratinoSens.png)
![Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C) 7 Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C)](https://www.scs-groups.com/wp-content/uploads/2024/12/Study-Design-of-In-Vitro-Skin-Sensitization-Dermal-Peptide-Reactivity-Assay-DPRA-OECD-442C.png)

![Study Design of In Vitro Eye Irritation [Bovine Corneal Opacity and Permeability (BCOP) Test] (OECD 437) 13 Study Design of In Vitro Eye Irritation [Bovine Corneal Opacity and Permeability (BCOP) Test] (OECD 437)](https://www.scs-groups.com/wp-content/uploads/2024/12/In-Vitro-Eye-Irritation-Bovine-Corneal-Opacity-and-Permeability-BCOP-Test.jpeg)