Study Design of EpiSkin-In Vitro Skin Corrosion (OECD 431)
The EpiSkin-In Vitro Skin Corrosion Test, based on the OECD Guideline 431, is a validated, non-animal alternative for assessing the corrosive potential of chemicals and mixtures. It utilizes reconstructed human epidermis (RhE) models, like EpiSkin™, to predict their ability to cause irreversible damage to the skin, similar to what occurs in humans.
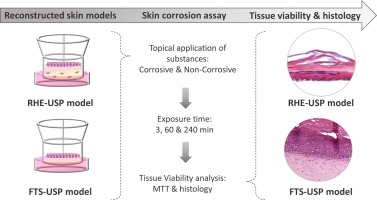
Test System:
EpiSkin™: A commercially available RhE model composed of non-transformed human keratinocytes cultured to form a multi-layered, differentiated epidermis closely resembling the structure and function of the human skin barrier.
Test Procedure:
- Test substance preparation: The test substance is prepared in appropriate concentrations and dilutions according to the OECD 431 protocol.
- Application to EpiSkin™: The test substance is applied topically to the surface of the EpiSkin™ model in specific volumes and exposure times.
- Incubation: The EpiSkin™ model is incubated under controlled conditions for a predetermined period, typically 24 hours, to allow the test substance to interact with the tissue.
- Viability assessment: After incubation, the viability of the EpiSkin™ cells is assessed using a vital dye, such as MTT or NRU, to determine the extent of cell damage caused by the test substance.
Scoring and Interpretation:
- The degree of cell damage is quantified based on the intensity of the vital dye uptake or other validated endpoints.
- A scoring system is applied to classify the test substance into different categories according to its corrosive potential:
- Non-corrosive: No significant cell damage observed.
- Corrosive: Extensive cell death and destruction of the RhE structure.
Borderline: Intermediate effects requiring further testing or data analysis.
Benefits:
- Reduced animal use: Eliminates the need for live animals, aligning with ethical considerations and animal welfare principles.
- Cost-effective: Generally faster and less expensive compared to traditional in vivo studies.
- Standardized protocol: Ensures international harmonization and data comparability.
- Predictive accuracy: Proven to be reliable in predicting skin corrosion potential with high sensitivity and specificity
Limitations:
- Limited tissue complexity: EpiSkin™ models lack certain components of the human skin, like blood vessels and nerves, which may influence the response to some substances.
- Not suitable for all chemicals: Certain highly volatile or reactive substances may not be adequately assessed using this method.
- Validation limitations: Although validated for specific categories of chemicals, ongoing research and validation efforts are necessary for broader applicability.
Additional Resources:
https://www.oecd-ilibrary.org/environment/test-no-431-in-vitro-skin-corrosion-reconstructed-human-epidermis-rhe-test-method_9789264264618-en
Linkedin Link
Medical Device Testing
Laboratory Testing

SCS Blog Category
Blog - ACUTE TOXICITY STUDIES
Blog - Biodegradability Testing – Chemical Impact
Blog - Environmental Fate & Toxicology Testing
Blog - In Vitro Studies
Blog - Laboratory Testing & Analysis
Blog - Medical Devices
Blog - OECD GLP
Blog - Scientific
Table of Contents
Toggle
