Blog - ACUTE TOXICITY STUDIES
 Acute Toxicity Studies
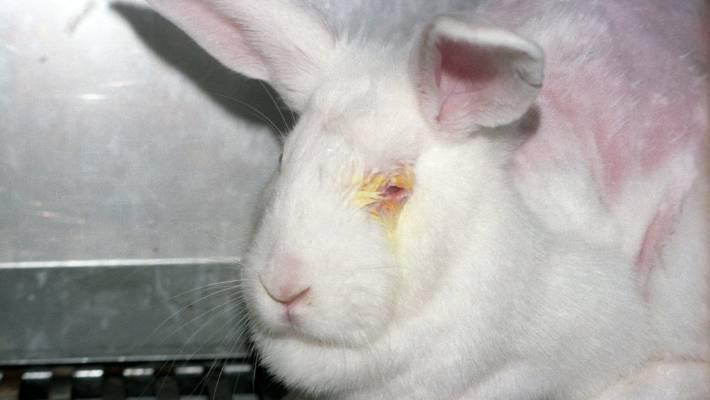
Acute Toxicity Studies Acute Toxicity Studies – Study Design of OECD Guideline 405: Acute Eye Irritation/Corrosion Study in Rabbits
Acute Toxicity Studies - Study Design of OECD Guideline 405: Acute Eye Irritation / Corrosion Study in Rabbits The OECD Guideline 405 outlines a standardized test for assessing the potential of a substance to irritate or corrode the eyes of
Read More Acute Toxicity Studies
Acute Toxicity Studies Acute Toxicity Studies – Study Design of OECD GLP TG 404 – Acute Dermal Irritation/Corrosion Study In Rabbits
Acute Toxicity Studies - Study Design of OECD GLP TG 404 - Acute Dermal Irritation/Corrosion Study In Rabbits The Acute Dermal Irritation/Corrosion Study in Rabbits (OECD 404) is a crucial test employed to assess the potential of a substance to
Read More Acute Toxicity Studies
Acute Toxicity Studies Acute Toxicity Studies – Study Design of Acute Inhalation Study in Rats (OECD 403)
Acute Toxicity Studies - Study Design of Acute Inhalation Study in Rats (OECD 403) The OECD Guideline 403 outlines the study design for assessing the acute inhalation toxicity of chemicals in rats. This information is crucial for ensuring the safety
Read More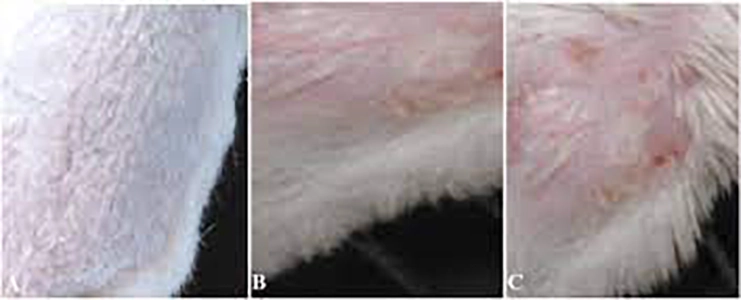 Acute Toxicity Studies
Acute Toxicity Studies Acute Toxicity Studies – Study Design of OECD Guideline 402: Acute Dermal Toxicity Study in Rats
Acute Toxicity Studies - Study Design of OECD Guideline 402: Acute Dermal Toxicity Study in Rats The OECD Guideline 402 outlines the study design for assessing the acute dermal toxicity of chemicals in rats. This information is crucial for ensuring
Read More Acute Toxicity Studies
Acute Toxicity Studies Acute Toxicity Studies – Study Design of OECD Guideline 425: Acute oral toxicity – up and down procedure
Acute Toxicity Studies - Study Design of OECD Guideline 425: Acute oral toxicity – up and down procedure The OECD Guideline 425 outlines the study design for the Acute Oral Toxicity – Up-and-Down Procedure, another method for assessing the potential harm
Read More Acute Toxicity Studies
Acute Toxicity Studies Acute Toxicity Studies – Study Design of OECD 423 Acute Oral
Acute Toxicity Studies - Study Design of OECD 423 Acute Oral The OECD 423 Acute Oral Toxicity Test Guideline is a stepwise procedure used to determine the acute oral toxicity of a substance in rodents, typically rats. It is designed
Read MoreLinkedin Link
Medical Device Testing
Laboratory Testing

SCS Blog Category
Blog - ACUTE TOXICITY STUDIES
Blog - Biodegradability Testing – Chemical Impact
Blog - Environmental Fate & Toxicology Testing
Blog - In Vitro Studies
Blog - Laboratory Testing & Analysis
Blog - Medical Devices
Blog - OECD GLP
Blog - Scientific

JOIN WITH MANY WHO HAVE BENEFITED WITH US
SCS Consulting Group

